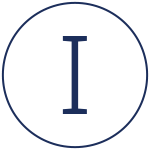
What Are Clinical Trials?
Clinical trials are human research studies used to assess new health questions. They are typically used to test new treatments and preventions for diseases and illnesses before the medications are made available to the public.
To uphold the integrity and ethics of Clinical research, all trials adhere to the globaly recognized Good Clinical Practice (GCP) standards.
GCP standards govern how clinical trials are to be conducted, performed, audited, and recorded. They also ensure that each volunteer’s rights, integrity, and confidential information are safeguarded.
Patient-Centered Care
Contact us and volunteer today to see if you qualify for one of our clinical research studies. Qualified volunteers may be compensated for time and travel.
BV Medical Research conducts Phase II - IV Trials
Benefits of participating in Clinical Trials

Compensation for Time and Travel

Access to New Treatments

Top Quality Patient Care and Attention

Patient Information Kept Confidential and Not Shared Without Permission

Ability to Withdraw from Study at Any Point
Be a part in accelerating ground-breaking research!
It‘s way more than just data and research. It’s about building your community and making the world a healthier place! Sign up for one of our clinical trials and get paid to help advancements in medicine.
Our Sites
Niagara Falls
Nearby Resources

Calgary
Nearby Resources

St. John's
Nearby Resources